The products produced by the dry method can be completely reacted. The reaction between the two would produce zinc stearate and water.
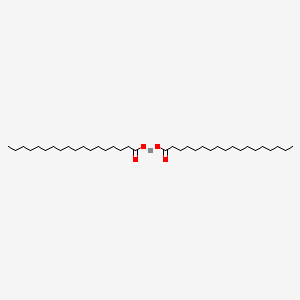
Zinc Stearate C36h70o4zn Pubchem
Water and zinc stearate were formed.

. Conveying liquid stearic acid by a pump allowing the liquid stearic acid to go through a flow meter for metering and then enter a zinc stearate reaction vessel stirring and heating. In the paints lacquers and varnishing industry Zinc Stearate is used as a sanding and flatting agent to improve the coating film feel to avoid the softening of the film caused by the temperature rise during mechanical polishing and to paste the sandpaper or grinding wheel to achieve a matting effect. Related but less extensive work has been carried out with ethyl stearate replacing the acid and with powders of zinc oxide MnO Mn 3 O 4 Mn 2 O 3 and MnO 2.
A Comprehensive Range Of Products and Enabling Technologies For Your Research. Emits acrid smoke and fumes of ZnO when heated to decomposition Hazardous Chemicals Desk Reference p. When reacting stearic acid and zinc hydroxide hydrochloric acid is further added to the mixture as a catalyst.
For zinc and zinc oxide the first reaction zone began at about 160 C and extended to 280290 C. Zinc Stearate is a fine soft odorless white powder with a molecular weight of 632 and the chemical formula Zn C 18 H 35 O 2 2. The reaction is carried out in an aqueous.
ZINC STEARATE is non-flammable but combustible. The invention belongs to the technical field of chemical engineering and particularly relates to high-quality zinc stearate prepared from glyceryl tristearate. The Hazard fields include special hazard alerts air and water reactions fire hazards health hazards a reactivity profile and.
For example the zinc stearate will melt during molding and be absorbed into the compound without leaving discoloration or defects on the surface of the final molded rubber part. The aqueous emulsion of zinc stearate is called zinc stearate emulsion. TABLE I FATTY ACIDS LENGTH LINEAR MELTPOINTC.
Buy Products At Sigma-Aldrich. 2 zinc oxide and a catalyst are added stepwise. Incompatible with oxidizing agents dilute acids.
Method of producing zinc stearate involves reacting stearic acid and zinc hydroxide with heating and intense stirring followed by heat treatment filtration drying and packaging. The make up is. Influence of Calcium Monostearate on Recrystallization of PP 110 112 114 116 118 120 122 124 DSC Peak No AOMST AONo MST.
It is widely used as a release agent for the production of many kinds of objects. Zinc stearate is produced from. The invention discloses a zinc stearate production technology.
DOT ID Guide. Figure 1 shows the molecular structure of zinc stearate. Ae Zinc stearate consisted of C16 and C18 fatty acid zinc salts as major components and small amounts less than 3 of C14 and C20 fatty acid zinc salts.
Ad Helping You To Solve the Tough Problems In Life-Science. Rubber polyurethane polyester processing system powder metallurgy. It is an activator for accelerated rubber sulfur vulcanization.
In cosmetics zinc stearate is a lubricant and thickening agent used to improve texture. Calcium Stearate Sodium Stearate Zinc Stearate Hydrotalcite 1000 ppm Acid Acceptor added Polypropylene 25 MF nominal powder 500 ppm phenolic AO700 ppm secondary phosphorus. Ismet Gokcel et al.
By monitoring features in the infrared spectra that are characteristic of the global conformation of the hydrocarbon chain it is shown that the double. The characteristic peaks of zinc techniques and DSC gave similar melting points. In this study the production of ZnSt2 using sodium stearate and zinc sulfate in a precipitation process and stearic acid and zinc oxide in a fusion process was investigated with.
As discovered in the early days of v. Control a certain temperature pressure and stirring speed to obtain stearate through reaction. A prominent feature of this compound is its very small particle size with a diameter of less than one micron.
These three products are all manufactured using the same raw materials and same base process but finished differently to provide a range of. From 280 to 375 C zinc stearate was present in the reaction products of the acid with both zinc metal and zinc oxide. Contexts in source publication.
The technology comprises the following steps. As a sanding agent Zinc Stearate fills. Dibasic zinc stearate Hydense Hytech Mathe Metallac Metasap 576 Octadecanoic acid zinc salt Petrac ZN-41 Stavinor ZN-E Stearates ACGIH Stearic acid zinc salt Synpro stearate Talculin Z Zinc distearate Zinc octadecanoate Zinc stearate ACGIHOSHA Acute Toxicity Data and References.
Dibasic zinc stearate Zinc distearate Zinc salt of stearic acid CAS No. At 600 C no acid soap was detected. Zinc chloride is strong Lewis acid and catalyses dehydrochlorination process but in the presence of calcium and barium stearates zinc chloride undergo ester exchange reaction thus regenerating the zinc stearate which may react with another molecule from hydrochloric acid or PVC this conclusion was reported by H.
Considering the molar masses of. These salts are produced from a reaction with stearic acid fatty acid and a metal oxide. Adding zinc oxide four times and reacting at a temperature of 160DEG C under a.
The stearate at 1540 and 1398 cm-1 were observed for the onset of melting was 120 C and 118 C and melting samples which were produced in precipitation process peak maxima were at 12517 C and 12577 C for ZnSt2 as shown in Figure 2a-c. It has a wide range of applications and can be used as a release agent color retention agent lubricant. B Equivalent number was calculated as mole of HCl per mole of zinc stearate.
A preparation technology of zinc stearate comprises the following steps that 1 glyceryl tristearate and water are hydrolyzed under the action of a catalyst and an antioxidant. ZINC STEARATE 201 ZINC STEARATE 201ND and ZINC STEARATE 325 are fusion reaction zinc oxide and fatty acid tallow based zinc stearates suitable for FDA CFR 21 indirect food applications. C Yield was calculated based on weight measurements.
These applications exploit its non-stick properties. D Reaction mixture remained insoluble throughout the reaction. Reaction between stearic acid and ZnO with zinc stearate formation 11 is represented in Figure 1.
The influence of a double bond in the middle of an otherwise flexible hydrocarbon chain on the melting of such assemblies has been investigated by comparing the melting behavior of zinc stearate and zinc oleate. The reactions of stearic acid with zinc and manganese powders have been studied from ambient temperature to 600 C mainly using differential scanning calorimetry and thermogravimetry. The reaction between the two would produce zinc stearate and water.

Zinc Stearate Formation And Vulcanization Mechanisms For Step 1 Download Scientific Diagram
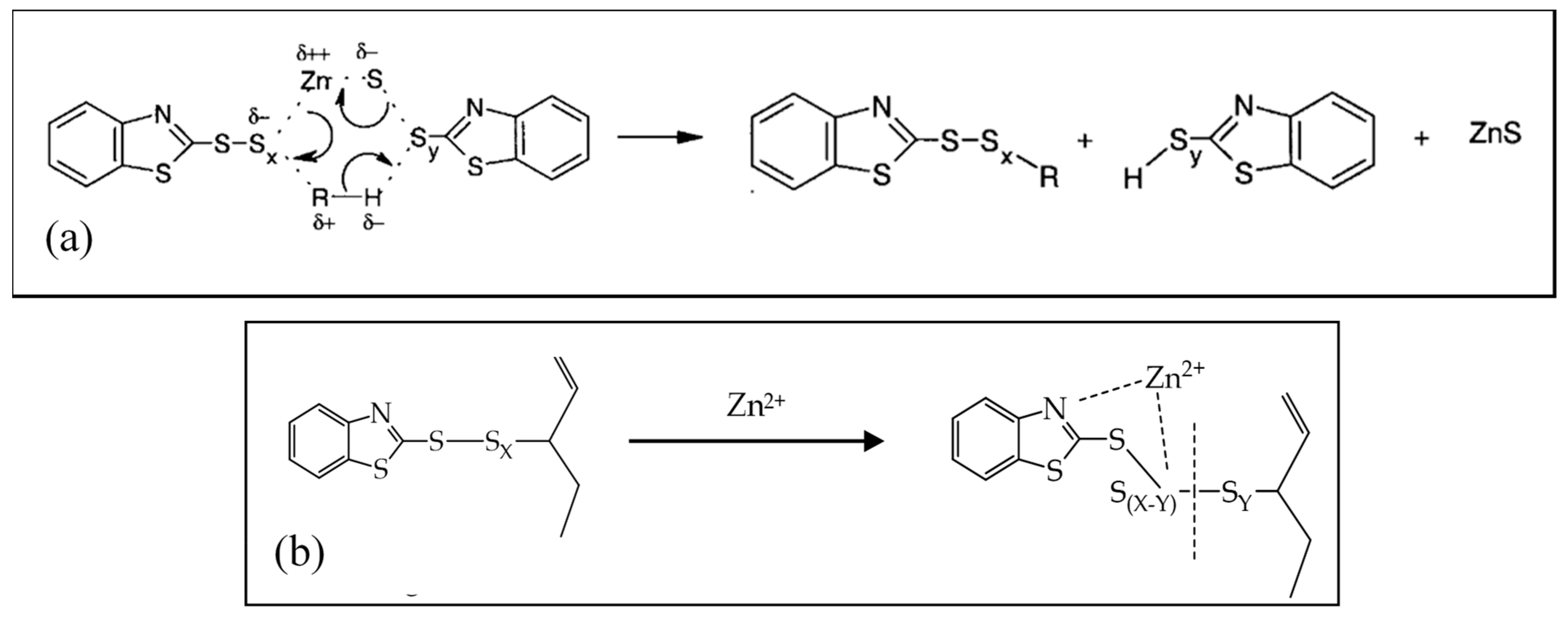
Catalysts Free Full Text Zinc Based Curing Activators New Trends For Reducing Zinc Content In Rubber Vulcanization Process Html

Effect Of Synthesized Zinc Stearate On The Properties Of Natural Rubber Vulcanizates In The Absence And Presence Of Some Fillers Sciencedirect

Influence Of Two Different Alcohols In The Esterification Of Fatty Acids Over Layered Zinc Stearate Palmitate Sciencedirect

Ion Exchange Between Silanol Groups And Zinc Stearate On Silica Surface 28 Download Scientific Diagram

Polymers Free Full Text Preparation And Characterization Of Waterborne Uv Lacquer Product Modified By Zinc Oxide With Flower Shape Html
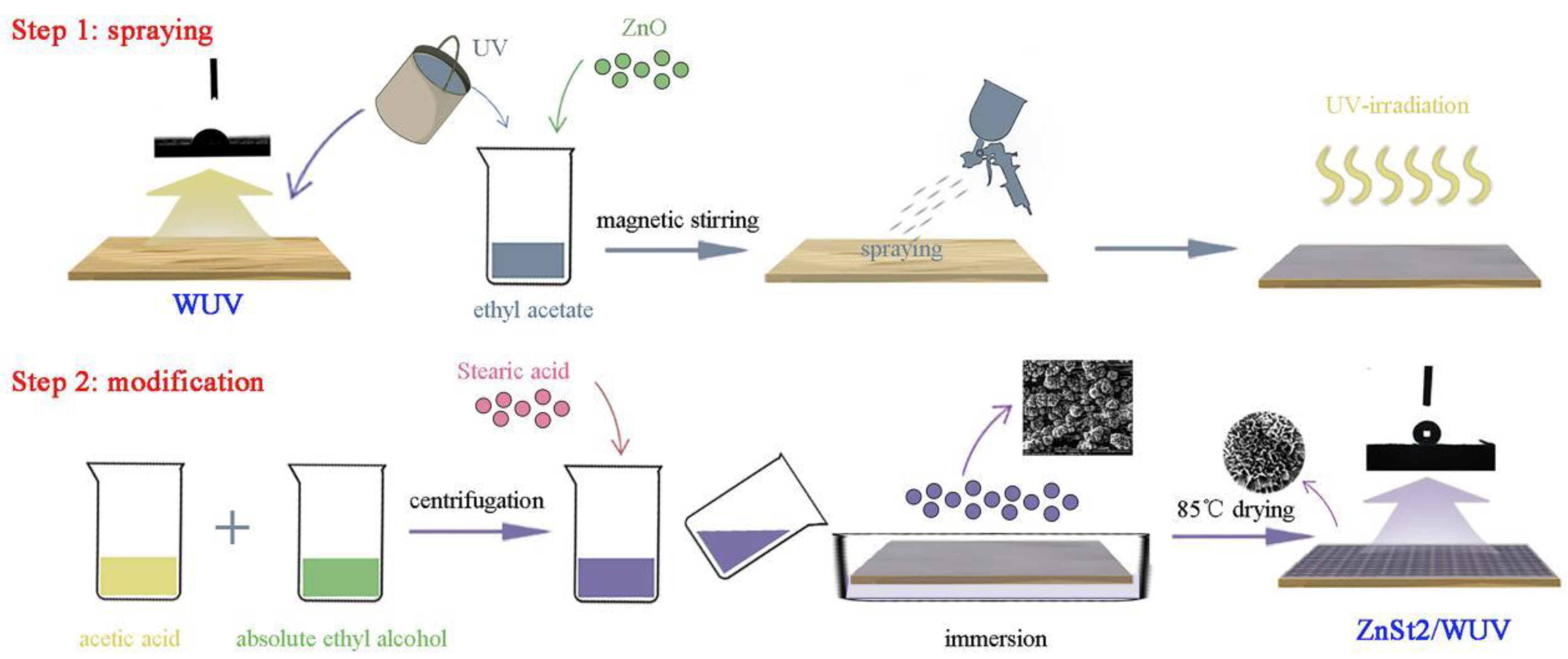
Polymers Free Full Text Preparation And Characterization Of Waterborne Uv Lacquer Product Modified By Zinc Oxide With Flower Shape Html

Pdf Zinc Stearate Production By Precipitation And Fusion Processes Semantic Scholar
Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram

Special Role For Zinc Stearate And Octadecene In The Synthesis Of Luminescent Znse Nanocrystals Chemistry Of Materials

Scielo Brasil Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract

Zinc Stearate Technical Grade 557 05 1

Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram

Tga Dsc Curves Of Zinc Stearate Reveal A Relative Thermal Equilibrium Download Scientific Diagram

Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram

Scielo Brasil Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract Influence Of Zno On The Properties Of Elastomeric Compositions And Their Leached Extract

